Four reasons why we need multiple vaccines for Covid-19
Having a range of Covid-19 vaccines available for people to use around the world is essential to bring the pandemic under control. Here’s why.

With several highly effective Covid-19 vaccines already in use, why is it important to keep working on and investing in the vaccines still in development?
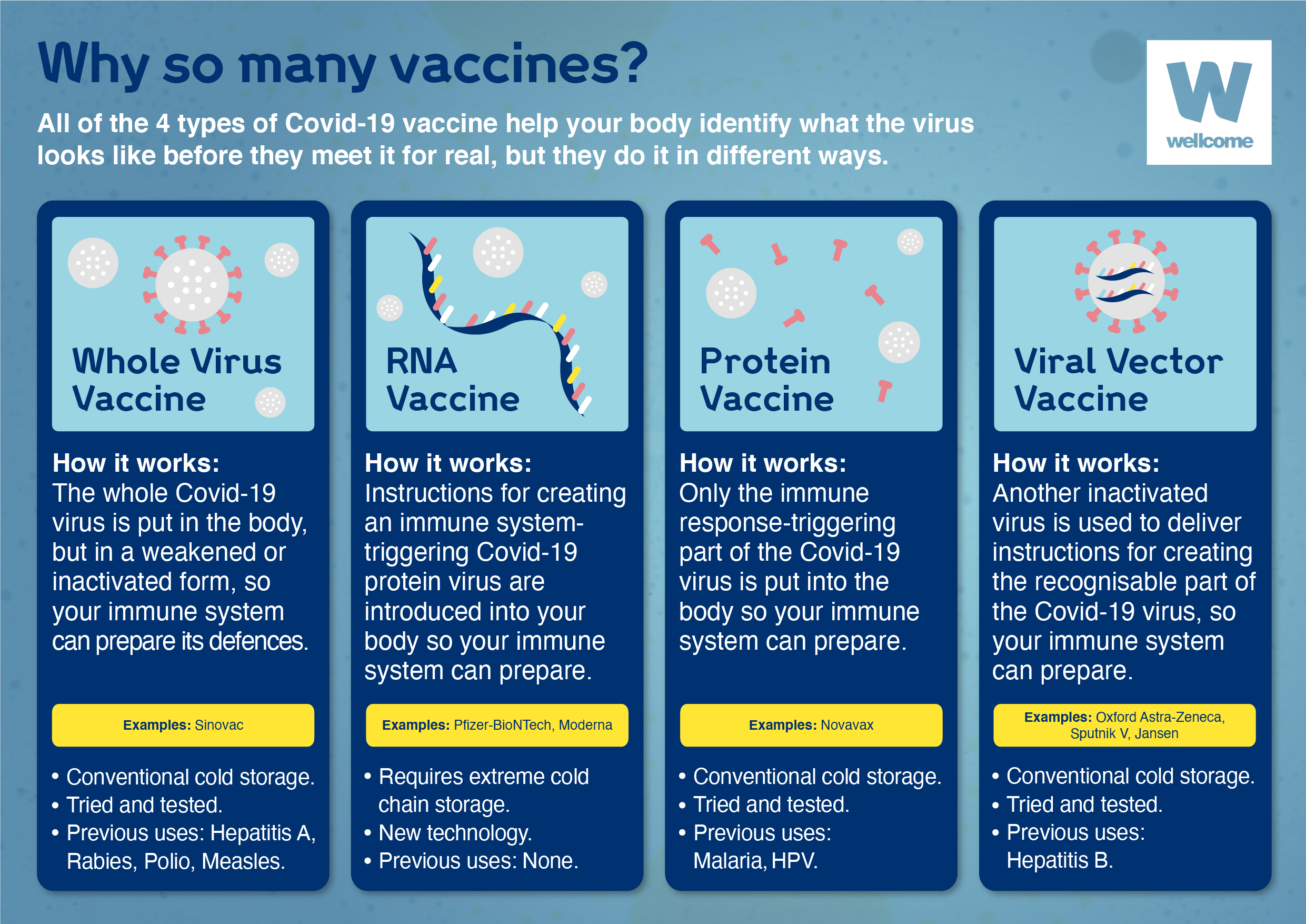
We need Covid-19 vaccines to work for a diverse range of people. At this stage, we are still collecting data to understand how effective the vaccines are for people of all ages, from different ethnic backgrounds, with different immune systems; and how well they work against different variants of the virus. We are also still investigating how long immunity might last for, and how effective the vaccines are at preventing transmission of the disease.
With questions like these remaining, we can’t rely on a small number of vaccines to help us respond to Covid-19.
And it might be that one vaccine is not as effective or suitable for everyone. We often need multiple vaccines for a disease to be able to protect different groups of people. For example, last winter the UK used four different flu vaccines. Which vaccine was given depended on a person’s risk group and how their immune system might respond – the vaccine given to a teenager with a robust immune system was not the same as that given to someone over the age of 65.
Vaccines are a bit like different painkillers: aspirin, paracetamol, ibuprofen: they all have the same goal, they just achieve it in slightly different ways.
If we have a varied Covid-19 vaccine portfolio, we can be confident we will find the right vaccine for every person.
To be able to get control of the virus we need to produce and roll out vaccines at a scale and speed never seen before. To meet the aim of vaccinating high-risk populations around the world by the end of 2021, we need at least 2 billion vaccine doses.
Companies are scaling up production as much as they can, for example Moderna have stated they hope to produce 800 million to 1 billion doses of their vaccine in 2021.
However, as we’ve already seen, there may be manufacturing issues and export delays which limit a vaccines’ availability. And because many countries have pre-ordered large quantities of the vaccines, a large share of the initial production is already spoken for.
By developing and investing in multiple vaccine candidates we stand a much better chance of having the volume of doses we need to help contain the virus.

We will need to get Covid-19 vaccines to everyone who needs them, wherever they are – from people living in metropolitan areas and cities, to rural populations in remote corners of the world.
Both the Pfizer-BioNTech and Moderna vaccines need to be stored and transported at exceptionally low temperatures: the Pfizer-BioNTech vaccine at -70C and the Moderna vaccine at -20C, to ensure they stay viable. In comparison, most childhood vaccines require storage at between 2C to 8C, similar to the temperature in a domestic fridge.
The requirements for cold storage and transportation will be difficult to meet in many locations, and no vaccine requiring such cold storage has ever been rolled out to so many countries, all at once.
In addition, both RNA vaccines currently have a relatively high cost per dose, although this could reduce once large-scale production and supply chains are in place. The news that other vaccines – such as the Oxford-AstraZeneca and Johnson & Johnson vaccines – cost significantly less has been widely welcomed. It is hoped that other highly effective vaccines will be offered at a lower cost.
Dosing creates an additional challenge. Most approved vaccines to date require two doses, spaced weeks apart. Developing effective single-dose vaccines will be critical to protect people for whom getting two doses might be difficult, such as those without access to regular healthcare, or refugees. Some manufacturers are currently developing single dose vaccines, and Johnson & Johnson’s has recently been approved for use. These vaccines could mark a turning point in global vaccine access.
These technical considerations mean it is vital that we continue to develop and invest in multiple vaccine candidates, which may be able to offer high levels of efficacy without the need for such cold storage or multiple doses.
To overcome the pandemic, people all around the world must have access to vaccines, treatments and tests. However, many of the vaccines being developed are being bought up by countries making deals directly with pharmaceutical companies for their populations.
As of April 2021, governments in high-income countries had purchased 56% of the world’s Covid-19 vaccine supply, despite representing only 16% of the global population.
And, although almost 2 billion vaccine doses have now been given across the world, most of them have been given in countries with the highest incomes.
The best and fastest way to achieve a more equitable distribution of vaccines lies in the hands of countries that have ordered more doses than they need. They can do this, alongside their own national rollouts, through the COVID-19 Vaccine Global Access Facility (COVAX), a multilateral effort to make sure that poorer countries are not frozen out of access to vaccines.
This article was originally published on 20 November 2020.
Did you find this content useful? Let us know your feedback at webmaster@wellcome.org.
Having a range of Covid-19 vaccines available for people to use around the world is essential to bring the pandemic under control. Here’s why.