Intellectual property and translation - our approach
A summary of our approach to Intellectual Property (IP). It provides advice on what you need to consider when you apply for funding, as well as during your grant, and when you commercialise Wellcome-funded IP.
This guide summarises our approach to Intellectual Property (IP). It provides advice on what you need to consider when you apply for funding, as well as during your grant, and when you commercialise Wellcome-funded IP.
We want to make sure that research outputs generated by Wellcome-funded science are translated into practical applications as quickly and effectively as possible.
This is line with our Constitution [PDF 153KB], which states that when funding research, 'It is expected that a large part of such research may lead to results that will benefit the life, health and well-being of humankind'.
We define research outputs broadly. They include patentable inventions, software, datasets, designs, materials (such as reagents and cell lines) and know-how.
The following overview applies when our standard grant conditions apply. It does not apply to:
- awards made to companies
- other funding made on bespoke terms by our Innovations team, usually done in the form of a funding agreement rather than a standard award letter.
In these cases, see the 'Working with our Innovations team' section.
We expect you to manage your research outputs in a way that will achieve the greatest health benefit. This might involve:
- translating inventions into new drugs or medical devices which benefit patients directly
- the development and dissemination of new research tools, software and materials, to help the research or translational efforts of others.
The methods used to generate impact from research outputs may also vary, but the chosen method must be based on an assessment of which one will lead to the greatest health benefit.
Researchers and their organisations must:
- make their outputs freely available to others (open access), or
- use intellectual property (IP) as a tool to protect and commercialise them.
When we assess grant applications, we look at the steps you've taken to benefit health through your previous research. This is as important to us as your publication history.
All applicants and organisations must read our:
- Grant conditions – section 8 (as well as condition 4.8 'Management of the Grant and reporting' for our annual IP reporting requirements)
- Intellectual property (IP) policy
- Consent and revenue and equity sharing policy.
If your research is likely to generate significant research outputs that will be of value to other researchers and users, you must complete the outputs management plan in your grant application. Read how to complete an outputs management plan for advice on what we're looking for and what factors to consider.
Collaborating with others on your research project
If you plan to collaborate with others on your research project, you must make sure that you comply with grant condition 8.1 in relation to the ownership and protection of IP.
The organisation administering your grant is expected to own all Wellcome-funded IP (apart from copyright in research articles which may be retained by the authors). Limited exceptions are allowed under grant condition 8.2, which enables access to proprietary materials and the background IP of others. In these cases, when dealing with others, your organisation must ensure that the funded research project can still deliver the anticipated public benefits.
Example:
If you're using a proprietary research tool provided by a company for other research purposes, it is acceptable to:
- share research data that you generate using that tool and which relates to the performance of that tool, and
-
allow that company to own any incidental improvements to the tool which happen to be made while using it.
However, all other results and IP generated from the use of the tool should be retained by the funded organisation, so that they can be published and/or commercialised in line with Wellcome’s policies.
Working with others outside academia
We encourage researchers to engage with people outside of academia to increase the impact of their research outputs. However, if this creates a potential conflict of interest, it will need to be managed appropriately.
If you have relationships with industrial partners, you should assess if you have a conflict that will need to be disclosed and managed, in line with our Conflicts of interest policy: Wellcome-funded researchers and commercial organisations. Examples include other (commercially funded) research collaborations or positions as consultants, scientific advisors or directors.
Researchers should immediately tell their research office/technology transfer office about the creation of any significant research outputs (for example, potentially patentable inventions, new software or new materials such as antibodies or cell lines).
Researchers should work closely with their organisation to determine whether to use IP as a tool to commercialise such outputs, or whether to instead make them freely available via open access.
If a significant research output has been created, and you have determined that the commercialisation of IP is the optimal route to deliver health benefit from it, you must comply with our:
- Grant conditions (condition 8.3), and
- Consent and revenue and equity sharing policy.
You can use your Wellcome grant to pay for the initial costs of protecting patents and registering designs. After this, your employer is responsible for all additional costs of protecting, maintaining and commercialising that IP throughout its lifetime.
Details of significant research outputs and IP creation must be included in your organisation's annual consolidated IP report to Wellcome. See the 'Reporting your IP to Wellcome' section for more information.
Getting consent
In most cases, you no longer need to get our consent before commercialisation. This applies to grants awarded under our standard grant conditions. We want to make it quicker and easier for organisations to get on with translating promising scientific discoveries.
Where our prior consent to commercialisation is still necessary, this will be stated in your award letter/funding agreement. Before you enter into any commercialisation agreement, we recommend that you check the specific terms under which Wellcome funded the project. This will help you, determine if you need to get our written consent first.
We can also withdraw this waiver of consent under our standard grant conditions if an organisation fails to meet our requirements. We check this each year when we review each organisation's consolidated IP report. If we withdraw the waiver, all future IP transactions by that organisation will need our prior written consent, until we feel comfortable enough with their activities to reintroduce the waiver.
Transactions
Any IP transactions must be consistent with our guidance on commercialisation agreements. In particular, the driving factor in the negotiation of any such agreement should be maximising public benefit via the potential for health impact, not revenue generation.
We particularly encourage organisations to include provisions in commercialisation agreements for equitable access for low- and middle-income countries, where appropriate.
All new transactions related to Wellcome-funded IP must be included in the organisation's annual consolidated IP report to Wellcome. See the 'Reporting your IP to Wellcome' section for more information.
Our revenue and equity share
In most cases, we apply a simple flat rate of 25% revenue and equity share for commercialisation of outputs resulting from our standard grants.
Our equity share is typically held by the relevant organisation and then treated as revenue when the organisation sells our shares along with its own.
Wellcome supports science to improve health, not to generate a financial return. However, we have a legal obligation to make sure that our funding is used for charitable purposes, not to financially benefit individuals or companies.
In the past, we met this obligation by taking our share of any revenue or equity generated when Wellcome-funded IP was commercialised, and then reinvested it for the public good. But we want to provide greater support to the translation of IP, and to recognise and reward those organisations that do so. Under our new arrangements, any university that generates revenue from translation can apply (annually when they submit their IP report), to keep our share to support future translational activities in their organisation, or to support equitable access.
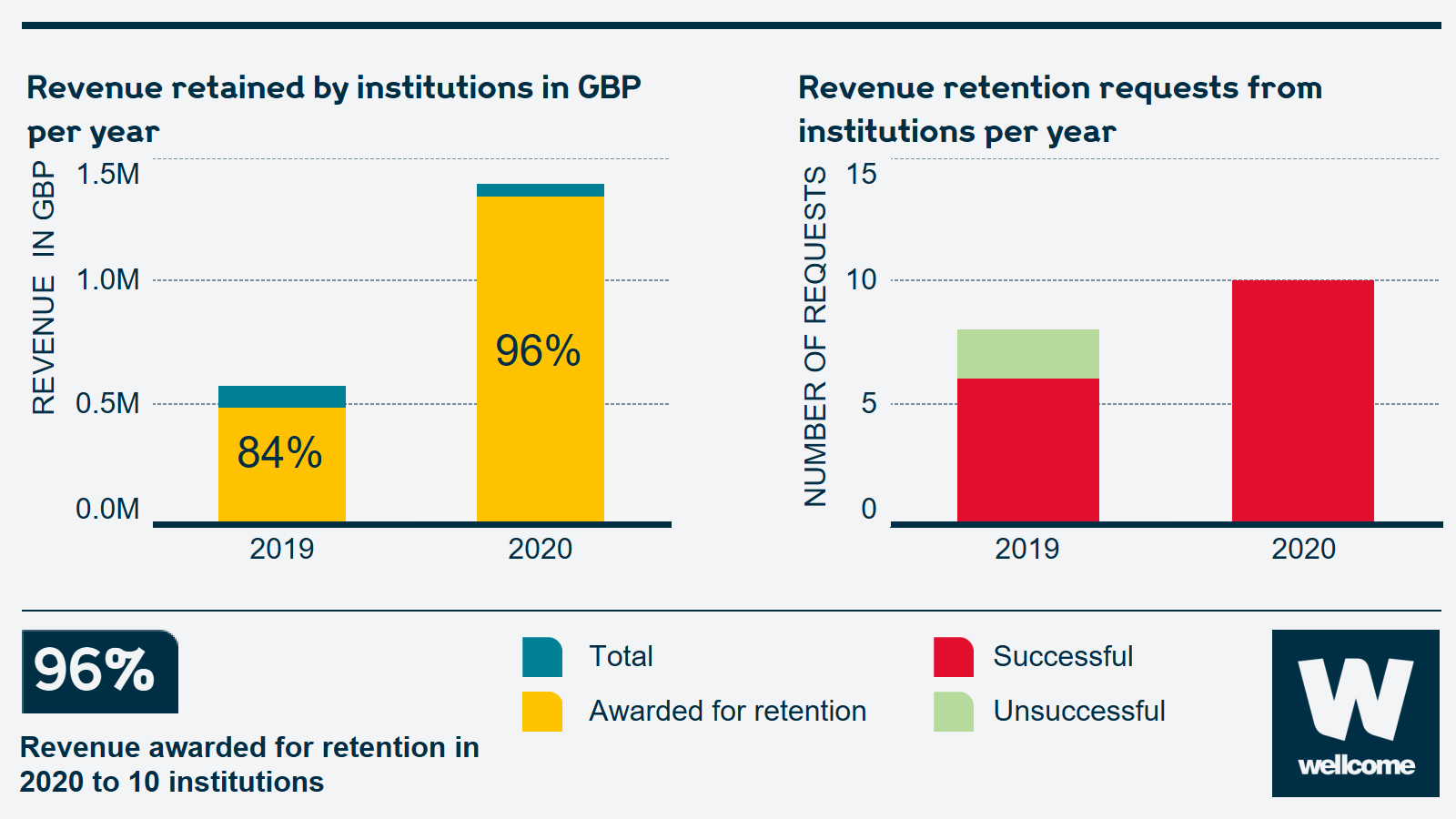
Information on the revenue that has been retained by organisations to date:

The revenue retained by institutions per year increased from around £500,000 in 2019 to more than £1 million in 2020. There was also a small increase in revenue retention requests from institutions per year in 2020 compared to 2021, and almost double the number of successful requests.
Your organisation is responsible for reporting IP creation and its subsequent sharing (open access) or its commercialisation to Wellcome, not individual researchers (Grant Condition 4.8). However, we expect researchers to work with their organisations to do so by keeping them updated on the creation of significant research outputs and their plans for using them.
Consolidated IP and commercialisation report
Your organisation must complete and submit a consolidated IP and commercialisation report by the end of February each year. This includes providing copies of the commercialisation agreements that you have entered into which relate to the activity set out in your report.
The way your organisation completes this report has changed. For the 2020-2021 reporting cycle onwards, they will need to use our electronic form.
Each November, we will send a customised link for the electronic form to each organisation's technology transfer team. If the technology transfer team at your organisation has not received this link by 1 December, contact innovationsoperations@wellcome.org.
Guidance on how to complete the electronic form is available in our quick start guide.
The report:
- should cover all IP-related activity for the previous calendar year and must detail Wellcome grant numbers, where known
- must include copies of the commercialisation agreements relevant to all IP-related activity
- must be submitted, even if only to report that there has not been any IP-related activity.
Organisations can only apply to retain Wellcome’s share of revenue received in the year covered by the report. If your organisation wishes to retain Wellcome’s revenue share, you must:
- include a revenue retention request in the report
- submit your report form to Wellcome by the February deadline – forms submitted after this date may result in your request being refused.
Your organisation must also complete Appendix 2 of the report – Our Revenue and Equity Sharing Agreement – if they entered into one or more commercialisation agreements related to Wellcome-funded IP in the year covered by the report.
Wellcome is deferring the submission of the 2021 IP and commercialisation report (which would ordinarily be due in February 2022) to February 2023.
Institutions are not required to submit their reports from the 2021 calendar year in February 2022, but will be expected to submit these alongside their reports from the 2022 reporting cycle in February 2023.
Wellcome will be in touch with institutions in due course to discuss the submission of the reports in 2023 (as highlighted in ‘Reporting your IP to Wellcome’ above).
Where our Innovations team has funded R&D projects – via either a bespoke funding agreement or a convertible loan (rather than on Wellcome’s standard grant conditions) – the specific provisions negotiated for that project will apply.
The protection and management of Wellcome-funded IP may be overseen by an IP management group and Wellcome’s prior consent to the commercialisation may be required. The revenue share may be different to the default 25%, but your organisation can still apply to retain it to support future translational activities or equitable access.
We produced a set of collective commitments to translation with the Royal Society, the Academy of Medical Sciences and the Royal Academy of Engineering in 2017. Following this initiative, we revised our policies, grant conditions and supporting documents relating to IP and translation, to help Wellcome funded researchers and organisations, and to support and reward translation.
Contact Grants Information Desk about:
- how to complete your grant application, including questions on outputs management plans
- how to comply with our IP policy.
Contact our Innovations team about:
- getting a customised link to the electronic consolidated IP and commercialisation report form
- how to complete and submit your IP report
- how to calculate revenue/equity share
- Innovations awards.