Intellectual property and translation - our approach
Get advice on what you need to consider when you apply for funding, as well as during your grant, and when you commercialise Wellcome-funded Intellectual Property.
This guide summarises our approach to Intellectual Property (IP). It provides advice on what you need to consider when you apply for funding, as well as during your grant, and when you commercialise Wellcome-funded IP.
Research outputs generated by Wellcome-funded science should be translated into practical applications as quickly and effectively as possible.
This is in line with our Constitution [PDF 153KB], which states that when funding research, 'it is expected that a large part of such research may lead to results that will benefit the life, health and well-being of humankind'.
We define research outputs broadly. They include patentable inventions, software, datasets, designs, materials (such as reagents and cell lines) and know-how.
The guidance on this page applies to awards made on our standard grant conditions. It does not apply to:
- awards made to companies
- other legacy schemes or awards which were made on bespoke terms by our Innovations team, usually done in the form of a funding agreement rather than a standard award letter.
In these cases, review the 'Legacy awards agreed with our Innovations team' section.
We expect you to manage your research outputs in a way that will achieve the greatest health benefit, while complying with our grant conditions and the policies we refer to on this page. This might involve:
- translating inventions into new drugs or medical devices which benefit patients directly
- developing and disseminating new research tools, software and materials, to help the research or translational efforts of others.
The methods used to generate impact from research outputs may also vary, but the chosen method must be based on an assessment that will lead to the greatest health benefit.
Researchers and their organisations must:
- make their outputs freely available to others (open access), or
- use intellectual property (IP) as a tool to protect and commercialise them.
When we assess grant applications, we look at the steps you've taken to benefit health through your previous research. This is as important to us as your publication history.
All applicants and organisations must read our:
- Grant conditions – section 8 (as well as condition 4.8 'Management of the Grant and reporting' for our annual consolidated IP and Commercialisation reporting requirements)
- IP policy
- Consent and revenue and equity sharing policy.
If your research is likely to generate significant research outputs that will be of value to other researchers and users, you must complete the outputs management plan section of your grant application. Learn how to complete an outputs management plan.
Collaborating with others on your research project
If you plan to collaborate with others on your research project, you must make sure that you comply with grant condition 8.1 in relation to the ownership and protection of IP.
The organisation administering your grant is expected to own all Wellcome-funded IP (apart from copyright in research articles which may be retained by the authors). This is important to avoid delaying the delivery of any public health impact. This can happen if various parties can’t reach agreement over the ownership of IP and how the IP in question will be later commercialised. Limited exceptions are allowed under grant condition 8.2, which enables access to proprietary materials and the background IP of others. In these cases, when dealing with others, your organisation must ensure that the funded research project can still deliver the anticipated public health impact. This is not an issue if no Wellcome-funded IP arises from the award.
This exception does not apply to ownership of IP by individual inventors because under charity law, public health impact must be prioritised and any private benefit must not be excessive.
If you're using a proprietary research tool provided by a company for other research purposes, you can:
- share research data that you generate using that tool, which relates to the performance of that tool, and
- allow that company to own any incidental improvements to the tool ( these are likely to be improvements which happen to be made while using the tool).
All other results and IP generated from use of the tool should be retained by the funded organisation, so that they can be published and/or commercialised in line with Wellcome’s policies.
Collaborators should not be able to veto conditions relating to the commercialisation of Wellcome-funded IP, but there is an expectation that they should be treated fairly, given their contribution to the project. Below are examples of some aspects that we would expect collaborators to agree within a collaboration agreement:
- access to arising datasets (for non-commercial research purposes) by collaborators is fair and reasonable;
- financial returns from any commercialisation (after deduction of our standard 25% revenue share) are distributed in an equitable way between collaborating institutions who contributed to the creation of the IP in question; and
- the lead organisation (as the commercialising party) should discuss the commercialisation plan with collaborators and update them on progress.
Working with others outside academia
We encourage researchers to engage with people outside of academia to increase the impact of their research outputs. However, if this creates a potential conflict of interest, it will need to be managed appropriately.
If you have relationships with industrial partners, you should assess if you have a conflict that will need to be disclosed and managed, in line with our Conflicts of interest policy: Wellcome-funded researchers and commercial organisations. Examples include other (commercially funded) research collaborations or positions as consultants, scientific advisors or directors.
Researchers should immediately tell their research office/technology transfer office about the creation of any significant research outputs (for example, potentially patentable inventions, new software or new materials such as antibodies or cell lines).
Researchers should work closely with their organisation to determine whether to use IP as a tool to commercialise such outputs, or whether to instead make them freely available through open access.
If a significant research output has been created, and you have determined that the best way to achieve health benefit is by commercialising the IP, you must comply with our:
- Grant conditions (condition 8.3), and
- Consent and revenue and equity sharing policy.
You can use your Wellcome grant to pay for the initial costs of protecting patents and registering designs. After this, your employer is responsible for all additional costs of protecting, maintaining and commercialising that IP throughout its lifetime.
Details of significant research outputs and IP creation must be included in your organisation's annual consolidated IP and Commercialisation report to Wellcome. Review the 'Reporting your IP to Wellcome' section for more information.
Getting consent
In most cases, you no longer need our consent before commercialisation. This applies to grants awarded under our standard grant conditions. We want to make it quicker and easier for organisations to translate promising scientific discoveries.
Where we still need to consent to commercialisation beforehand, this will be stated in your award letter/funding agreement. Before you enter into any commercialisation agreement, we recommend you check the specific terms under which Wellcome funded the project. This will help you determine if you need to get our written consent first.
We can also withdraw this waiver of consent under our standard grant conditions if an organisation fails to meet our requirements (including failing to submit their annual IP and commercialisation report). We check this each year when we review each organisation's annual consolidated IP and Commercialisation report. If we withdraw the waiver, all future IP transactions by that organisation will need our prior written consent, until we feel comfortable enough with their activities to reintroduce the waiver.
Transactions
Any IP transactions, such as licences, options, assignments, material transfer or evaluation agreements, must be consistent with our guidance on commercialisation agreements. The driving factor when negotiating these agreements should be maximising public benefit to achieve the maximum health impact, not generating revenue.
Wellcome expects a heavy focus on equitable access to ensure the maximum health impact is achieved. We expect organisations to include provisions in commercialisation agreements for equitable access for low- and middle-income countries where appropriate. The GHIAA MAPGuide may be a good starting point for institutions to review various equitable access clauses and strategies that may be suitable for them to use. Working with patent pools (such as the Medicines Patent Pool) to licence Wellcome-funded IP can be an effective method of facilitating equitable access.
All new transactions related to Wellcome-funded IP must be included in your organisation's annual consolidated IP and Commercialisation report to Wellcome. Review the 'Reporting your IP to Wellcome' section for more information.
Our revenue and equity share
In most cases, we apply a flat rate of 25% revenue and equity share where outputs of our standard grants are commercialised.
Our equity share is typically held by the relevant organisation on trust for us, and then treated as revenue when the organisation sells our shares along with its own.
Although we support science to improve health, we have a legal obligation to make sure that our funding is used for charitable purposes – not to financially benefit individuals or companies.
We want to provide greater support to translation. We are doing this by rewarding organisations that translate Wellcome-funded IP to achieve a health benefit.
Any university that generates revenue from translation can apply to keep our share to support new translational activities. These activities should be underpinned by a focus on improving equitable access. Organisations can do this when they submit their annual consolidated IP and Commercialisation report to Wellcome.
Revenue that has been retained by organisations to date:
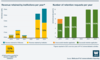
The revenue retained by institutions per year has increased from a total of around £500,000 to nearly £2.7 million. The number of institutions which applied to retain Wellcome’s share of revenue has also increased, from 8 in 2019 to 12 in 2024, all of which were successful.
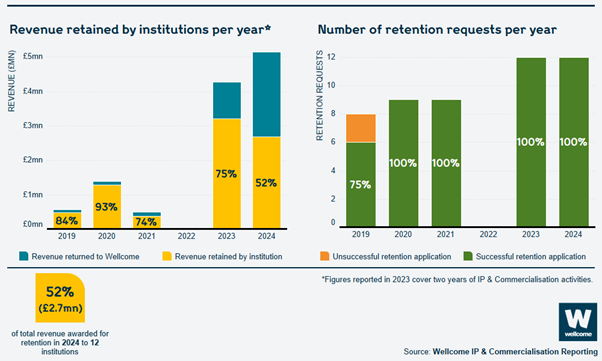
The revenue retained by institutions per year has increased from a total of around £500,000 to nearly £2.7 million. The number of institutions which applied to retain Wellcome’s share of revenue has also increased, from 8 in 2019 to 12 in 2024, all of which were successful.

This year we achieved 98% compliance overall of reports submitted. Over £5.1 million in revenue was due to be returned to Wellcome, and we allowed institutions to retain nearly £2.7 million of this. 12 institutions retained Wellcome’s share of revenue that they had generated and 7 institutions returned Wellcome’s share (see ‘Applying to retain revenue’).
In the 2024 reporting cycle, we learned of:
- 76 new transactions (IP assignments, licences) including 15 new equity transactions (spin-outs)
- 107 invention disclosures
- 134 patent applications
- 183 granted patents
- 45 activities relating to other IP
Your organisation is responsible for reporting IP creation and its subsequent sharing (open access) or its commercialisation to Wellcome (Grant Condition 4.8). We expect researchers to work with their organisations to support this by keeping them updated on the creation of significant research outputs and their plans for using them.
IP and Commercialisation report
Your organisation must complete and submit a consolidated IP and Commercialisation report by the end of February each year. This includes providing unredacted copies of commercialisation agreements that you have entered into, which relate to the activity set out in your report. This is a condition of our funding and maintains your institution’s prior consent waiver (see Grant Condition 8.3(b)).
Each November, we will send a unique link to each organisation's technology transfer team so they can complete the electronic form. If the technology transfer team at your organisation has not received this link by 1 December, contact IPCommercialisation@wellcome.org. Please use this short form to ensure your organisation’s contact details are kept up to date, particularly if key contacts for the reporting process leave your organisation.
Guidance on how to complete the electronic form is available in our IP and Commercialisation Quick Start Guide.
The report:
- should cover all IP-related activity for the previous calendar year and must detail Wellcome grant numbers, where known
- must include copies of the commercialisation agreements relevant to all IP-related activity
- must be submitted, even if you’re only saying there hasn’t been any IP-related activity.
It is your organisation’s responsibility to ensure that all information contained in your report is accurate to the best of your knowledge.
If you do not provide full, unredacted copies of agreements in future cycles, your institution's prior consent waiver will be revoked. You would then need to obtain our written consent to each proposed transaction before it can be finalised. This will likely lead to contracting delays.
Overseas organisations are responsible for ensuring they account for any relevant taxes in country in which their organisation is based. Wellcome is not responsible for any such taxes. You should seek specialist advice if you have any questions.
Review the general feedback we provided to all submitting organisations this year.
We saw many great clauses, including:
- clearly carving out which party owns IP and improvements where multiple parties are contributing their IP for the purposes of commercialisation;
- including low- and middle-income countries (LMICs) within the development milestones in order to ensure that the IP in question is made available in those territories at an affordable price within an appropriate timeframe;
- setting out the circumstances in which IP would be abandoned (to avoid licensees sitting on an exclusive licence where they are not doing anything with it);
- clear consequences where there are disagreements around commercialisation, for example in some cases, expert review. As a reminder, development of the IP should be a priority to ensure the maximum health impact is achieved;
- in an exclusive, field restricted, licence we saw a good clause that allowed the field to be carved up. If the licensor sees an opportunity to use the licensed IP to develop products in the field that the existing licensee does not wish to explore, then the institution could license the IP to another party. This helps maximise the health impact of the IP rather than it sitting exclusively with one party whose plan to commercialise it is more limited.
Wellcome is increasingly focusing on equitable access to ensure the maximum public health benefit is achieved.
Whilst we saw some engagement with this issue in commercialisation agreements, a much stronger focus on equitable access is required overall.
We saw a good clause which provided for the outputs to be made available to LMICs in accordance with Wellcome's equitable access statement. This included having an appropriate global access plan in place and ensuring that any economic barriers in place were low.
Other mechanisms we have seen work in the past are:
- guaranteed supply volumes or price caps in certain territories;
- priority access to products for countries involved in research or clinical trials;
- use of commercially reasonable or best efforts to keep barriers to access as economically low as possible whilst remaining sustainable for the licensee;
- clear consequences of what happens if the IP in question is not made available as the equitable access clause provides within a set timeframe (for example, 3‐5 years as appropriate for the technology in question). For example, see ‘Step‐in rights’.
We appreciate that this can be a challenging topic to address with licensees, but where the technology in question has application to LMICs, Institutions must ensure that it is not just patients in the developed world that will benefit.
Institutions can refer to the GHIAA MAPGuide to review guidance notes and see examples of various provisions & strategies that may be suitable for use in their licences.
Wellcome will monitor this closely in future cycles with a view to seeing an improvement. Failure to address equitable access (and instead focussing solely on maximising financial return) is in our opinion the issue most likely to lead to our removing an institution’s consent waiver in future years.
In the agreements with spin‐outs that we saw, it was not always clear that it was a spin‐out. This should ideally be made clear at the outset of the agreement. Diligent efforts clauses with spin outs were also weak ‐ as a reminder, we would expect the same diligence requirements should apply to a spin‐out as they would to any other third party licensee. A stronger focus on future health impact is also required and better use of objective obligations. For example, what does the spin‐out need to achieve in terms of technology development and/or moving the product towards market, and by when in order to retain control of the IP?
Many licences did not include recitals ‐ even brief recitals with some background on the agreement would be preferred for context, and some IP agreements were missing basic boilerplate provisions such as termination clauses.
As a reminder, submission of these reports is not optional ‐ it is a condition of our funding (Grant Condition 8.3(b)) and for retention of the prior consent waiver for your institution.
This year we achieved 100% compliance with submission of the reports, though some institutions failed to report on time. Please ensure that your institution’s contact details are up to date so that you can receive the bespoke reporting link for the next reporting cycle without delay. Failure to keep these updated will result in us sending the link to an inactive email address. If we do not receive a response despite chasing, this could result in your institution’s consent waiver ultimately being revoked.
We cannot justify retaining the prior consent waiver if we cannot meaningfully review the transactions you enter into and assess whether their terms are reasonable and in accordance with our policies and guidance. This is difficult if you a) do not supply agreements on the basis that they are confidential or b) redact key sections of the agreement, as some institutions have tried to do. The annual survey details the confidentiality terms that apply. You can share an extract of this with your licensees if necessary. Some institutions are also starting to insert provisions in their licences to inform licensees that their funder(s) will review licences, either a more general exception or by naming Wellcome. We would encourage you all to do that as a matter of course in any transactions relating to Wellcome‐funded IP.
For example:
The Licensor may disclose Confidential Information of the Licensee to any Third Party:
(i) to whom the Licensor is, at the Effective Date, under a legal or contractual obligation to disclose such information, or
(ii) who was involved in the development of the Licensed Intellectual Property Rights/Licensed Products/Services [as defined in the agreement] including the creation, invention or funding of the Licensed Intellectual Property Rights/Licensed Products/Services. The Licensor shall use reasonable endeavours to procure that the Confidential Information is treated in accordance with this Agreement.
Failure to supply unredacted agreements in their entirety in future cycles will result in your institution's prior consent waiver being revoked. You would then need to obtain our prior written consent to each proposed transaction before it can be finalised, which we know from prior experience is likely to lead to delays in contracting.
Finally, we note that a few institutions returned revenue to Wellcome this year. We are keen for institutions to retain our share of the revenue that they have generated, where they can put it to good use by boosting translational or equitable access activities aligned to our mission of improving human health. Please note that the retained revenue does not need to be put towards projects arising from or related to previous Wellcome funding. If you would like to discuss please contact us.
Once again thank you for the effort that has been put into compiling these reports. We look forward to reviewing the reports as part of the next cycle. Please look out for your invitation in early November. We will also publish a copy of this guidance, and updates on past reporting cycles, where we outline our approach to IP and Translation.
This feedback has been collated in response to previous submissions.
Well-drafted clauses include:
- clearly carving out which party owns IP and improvements where multiple parties are contributing their IP for the purposes of commercialisation;
- in most cases, clear definitions of terms such as ‘commercially reasonable efforts’;
- including low- and middle-income countries (LMICs) within the development milestones in order to ensure that the healthcare products in question are made available in those territories at an affordable price within an appropriate timeframe;
- setting out the circumstances in which IP would be abandoned or revert to the licensor (to avoid licensees sitting on an exclusive licence where they are not doing anything with it);
- clear consequences where there are disagreements around commercialisation, such as expert review. Development of the IP should be a priority to ensure the maximum health impact is achieved. The use of independent experts to make diligence requirements more enforceable is encouraged;
- allowing the field to be carved up in an exclusive, field restricted licence. If the licensor sees an opportunity to use the licensed IP to develop products in the field that the existing licensee does not wish to explore, then the institution could license the IP to another party. This helps maximise the health impact of the IP rather than it sitting exclusively with one party whose plan to commercialise it is more limited.
Wellcome expects a heavy focus on equitable access to ensure the maximum public health benefit is achieved.
Whilst we have seen some engagement with this issue in commercialisation agreements, a much stronger focus on equitable access is required overall.
Clauses which provide for the outputs of a grant to be made available to LMICs in accordance with Wellcome's equitable access statement are preferable. This includes having an appropriate global access plan in place and ensuring that any economic barriers in place were low. We have also seen specific reference to meeting Wellcome’s objectives and an institution’s access statement. The institution’s access statement contained examples of how it would develop creative licensing strategies to prioritise affordable access. This goes a step further to help ensure access commitments can be achieved.
Other mechanisms we have seen work in the past are:
- guaranteed supply volumes or price caps in certain territories;
- priority access to products for countries involved in research or clinical trials;
- use of commercially reasonable or best efforts to keep barriers to access as economically low as possible whilst remaining sustainable for the licensee;
- clear consequences of what happens if the IP in question is not made available as the equitable access clause provides within a set timeframe (for example, 3‐5 years as appropriate for the technology in question). For example, see Step-in rights below.
We appreciate that this can be a challenging topic to address with licensees, but where the technology in question could be useful in LMICs, institutions must ensure that it is not just patients in the developed world that will benefit.
Refer to the GHIAA MAPGuide for useful guidance notes and to see examples of various provisions & strategies that may be suitable for use in an institution’s licences.
Wellcome will monitor this area closely in future cycles with a view to seeing an improvement. Failure to address equitable access (and instead focussing solely on maximising financial return) is the most likely reason for us to remove an institution’s consent waiver in future.
Our goal is to ensure that the Wellcome funded IP in question leads to a public health benefit without significant delays. Therefore, Wellcome‐funded IP must not be maintained solely as a barrier to the research, development and commercialisation activities of others. Some agreements have not contained provisions to address what happens if commercialisation is held up.
For example, you should have the necessary reversion rights to commercialise (in the context of exclusive licences) if the licensee doesn’t do so, within a reasonable time for that IP. Refer to Wellcome’s step-in rights for more information, as referred to in our standard grant conditions.
We would first expect you to reclaim the IP (for example, by the exclusive licence terminating or becoming non‐exclusive) if it wasn’t being actively developed by your licensee. Ensure you preserve your rights to do this.
In the agreements with spin‐outs that we’ve seen previously, it was not always clear that it was a spin‐out. This should ideally be made clear at the outset of the agreement. Diligent efforts clauses with spin-outs were also weak ‐ we would expect the same diligence requirements should apply to a spin‐out as they would to any other third party licensee.
A stronger focus on future health impact is also required and better use of objective obligations. For example, what does the spin‐out need to achieve in terms of technology development and/or moving the product towards market? And when does this need to happen in order to retain control of the IP?
- Recitals: We recommend inserting brief recitals with some background on the agreement to provide context.
- Termination: Ensure IP agreements include basic boilerplate provisions such as termination clauses.
Each report enables us to assess whether the terms are reasonable and in accordance with our policies and guidance. This is difficult if you:
a) do not supply agreements on the basis that they are confidential, or
b) redact key sections of the agreement. The annual survey contains confidentiality provisions, which you can share an extract of, with your licensees, if necessary.
Some institutions are inserting provisions in their licences to inform licensees that their funder(s) will review licences, sometimes specifically naming Wellcome. We would encourage all licences to include this in any transactions relating to Wellcome‐funded IP. For example: The Licensor may disclose Confidential Information of the Licensee to any Third Party:
(i) to whom the Licensor is, at the Effective Date, under a legal or contractual obligation to disclose such information, or
(ii) who was involved in the development of the Licensed Intellectual Property Rights/Licensed Products/Services [as defined in the agreement] including the creation, invention or funding of the Licensed Intellectual Property Rights/Licensed Products/Services.
Organisations can only apply to retain Wellcome’s share of revenue received in the year covered by the report. If your organisation wants to retain Wellcome’s revenue share, you must:
- include a revenue retention request in the report
- submit your report form to Wellcome by the February deadline. Forms submitted after this date may result in your request being refused.
We are keen for institutions to retain our share of revenue that they generate. If your application to retain revenue is successful, the retained revenue can be spent on any projects as long as they focus on new translational activity and are underpinned by considerations to improve equitable access to healthcare interventions, particularly in low- and middle-income countries. The project does not need to be Wellcome-funded or linked to the original project that generated the revenue. While we would prefer for retained revenue to be used towards areas that align with Wellcome’s strategic goals, even if that work was originally funded elsewhere, this is not a requirement.
You should provide as much detail as possible on the proposed use of the retained funds in your application. If you would like to change the proposed use at any point following a successful application, you must inform us beforehand.
Your organisation must also complete Appendix 2 of the report – Our Revenue and Equity Sharing Agreement – if they entered into one or more commercialisation agreements related to Wellcome-funded IP in the year covered by the report.
Retained revenue can be added to an institutional fund for IP and patent costs but should not replace or substitute the core funds available at that institution.
Wellcome’s IP and revenue sharing policies will not typically apply to activities funded indirectly through retained revenue, however we retain the right to request that our conditions flow down in certain circumstances (for example, depending on the value retained and the proposed work).
If you are expecting large revenue amounts, especially those which are unusual in your organisation’s history of translational activities, please bring this to our attention as soon as possible and before submitting a revenue retention request. This will help shape your application to retain revenue and reduce the risk of an unsuccessful application.
For large retention applications, we also encourage institutions to describe how any retained revenue will be matched from other sources, to maximise the potential benefits.
Early discussion of unusually large transactions may also allow us to consider whether an extended spend period is required for the amount in question. Retained revenue must usually be at least committed within one year of the revenue retention request being approved. Organisations are informed about the outcome of their revenue retention requests annually around June. The spend report is then due at the end of the following reporting period in December. In practice, this results in around 18 months to spend any retained revenue. Wellcome will notify organisations when spend reports are due.
Depending on the proposal, we may grant a longer spend period. This is at our complete discretion and would be an exception. We reserve the right to:
- enforce limits on retained amounts and spend periods – for example, if we have concerns about an organisation’s ability to use the revenues effectively; and
reject exceptionally large amounts (this will depend on what is unusually large for the organisation in question, based on their history of translational activity).
Individual transactions below £10,000 relating to material transfers do not need to be reported. Retained income in this category should still be used to support translational activity and equitable access. We retain the right to review or audit the use of these amounts.
Individual transactions below £10,000 relating to anything else should be reported as usual.
Ensure your institution’s contact details are up-to-date so you can receive the bespoke link for the next reporting cycle. If you do not keep these updated, we may end up sending the link to an inactive email address. If we do not receive a response after following up, this could result in your institution’s consent waiver ultimately being revoked and your institution missing the opportunity to retain Wellcome’s revenue share. You can add more than one contact email address.
Where our Innovations team funded Research and Development projects – through either a bespoke funding agreement or a convertible loan (rather than on Wellcome’s standard grant conditions) – the specific provisions negotiated for that project will apply.
The protection and management of Wellcome-funded IP may be overseen by an IP management group and Wellcome’s prior consent to the commercialisation may be required. The revenue share may be different to the default 25%, but your organisation can still apply to retain it to support future translational activities with a view to improving equitable access.
Contact Funding Information Desk about:
- how to complete your grant application, including questions on outputs management plans
- how to comply with our IP policy.
Contact our IP Commercialisation inbox about:
- getting a customised link to the electronic consolidated IP and commercialisation report form
- how to complete and submit your IP report
- how to calculate revenue/equity share